Gas Adsorption in an Isostructural Series of Pillared Coordination Cages
Eric J. Gosselin, Gregory R. Lorzing, Benjamin A. Trump, Craig M. Brown and Eric D. Bloch
ChemComm
Issue 49, 2018
Department of Chemistry & Biochemistry, University of Delaware, Newark, USA
E-mail: edb@udel.edu
Center for Neutron Science, Department of Chemical and Biomolecular Engineering, University of Delaware
Center for Neutron Research, National Institute of Standards and Technology, Gaithersburg, USA
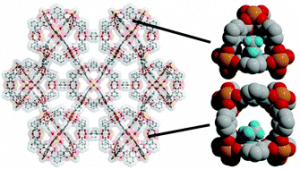 The synthesis and characterization of two novel pillared coordination cages is reported. By utilizing 1,4-diazabicyclo[2.2.2]octane (dabco) as a pillar with increased basicity as compared to pyrazine or 4,4′-bipyridine, a stable copper-based material was prepared. Extending this strategy to iron(II) afforded an isostructural material that similarly retains high porosity and crystallinity upon solvent evacuation. Importantly, the iron solid represents a rare example of porous iron paddlewheel-based metal–organic material that is stable to solvent evacuation. Neutron powder diffraction studies on these materials indicate the triangular and square windows of the cage are prime ethane and ethylene adsorption sites.
The synthesis and characterization of two novel pillared coordination cages is reported. By utilizing 1,4-diazabicyclo[2.2.2]octane (dabco) as a pillar with increased basicity as compared to pyrazine or 4,4′-bipyridine, a stable copper-based material was prepared. Extending this strategy to iron(II) afforded an isostructural material that similarly retains high porosity and crystallinity upon solvent evacuation. Importantly, the iron solid represents a rare example of porous iron paddlewheel-based metal–organic material that is stable to solvent evacuation. Neutron powder diffraction studies on these materials indicate the triangular and square windows of the cage are prime ethane and ethylene adsorption sites.


