Cathy Wu, Craig Brown and Anderson Janotti
Cathy Wu, Craig Brown and Anderson Janotti make list of top influencers
Engineers at the University of Delaware do research that garners attention from scientists and engineers around the world, and three faculty members in the College of Engineering were recently named to the Clarivate Analytics list of Highly Cited Researchers for 2018. This list identifies scholars whose publications are in the top 1% for citations by other researchers via Web of Science, a scientific citation indexing service. Researchers can be cited for top performance in their field or for Cross-Field impact, a new category this year. Read on for more about UD engineering’s highly cited academics.
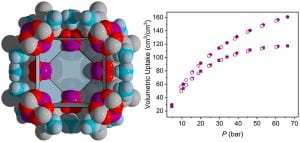
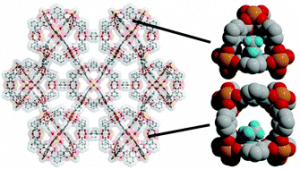
Craig M. Brown is a staff chemist at the National Institute of Standards and Technology (NIST) center for Neutron Research and an adjunct professor through UD’s Center for Neutron Science, which was founded in 2007. Under a cooperative agreement with NIST, UD’s Center for Neutron Science advances the field of neutron scattering by developing new techniques, applying these techniques to new applications, and training the next generation of neutron scientists. Brown, who studies the structure and dynamics of novel materials, made the 2018 Highly Cited Researchers list in the Cross-Field category. Among his most cited works are papers on molecular adsorption for energy efficient industrial separations, and hydrogen storage in materials, which hold promise in applications for cleaner energy and automotive technology. Brown has published more than 175 peer-reviewed papers, which have garnered more than 12,000 citations. His work has an h-index of 50, and an i10-index of 144 based on Google Scholar.
View College of Engineering News article